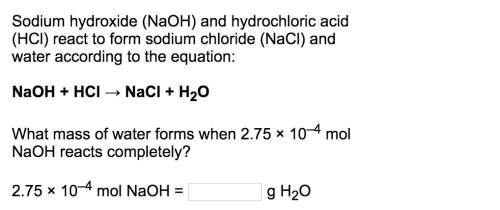
Naoh + hcl → nacl + h2o
what mass of water forms when 2.75 × 10–4 mol naoh reacts completely?<...

Chemistry, 26.08.2019 10:30 jakalenn2018
Naoh + hcl → nacl + h2o
what mass of water forms when 2.75 × 10–4 mol naoh reacts completely?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
Questions












Mathematics, 13.08.2019 01:30

Mathematics, 13.08.2019 01:30



History, 13.08.2019 01:30

Biology, 13.08.2019 01:30

English, 13.08.2019 01:30

History, 13.08.2019 01:30



