Achemist is using a solution of hno3 that has a ph of 3.75.
what is [h+] for the solutio...

Chemistry, 10.10.2019 21:30 jholland03
Achemist is using a solution of hno3 that has a ph of 3.75.
what is [h+] for the solution?
⇒ *1.78* × 10^n m
n = *-4*
okay so i don't get chemistry and i'm getting a tutor eventually. the blank is what i had to fill in and the arrows are pointing to a number in as-tricks these are the correct answer. however i wish for an explanation. on where this number came from i know i'm suppose to use a calculator and i know i use the "log" button however what do i throw in it. (err 75 points instead of cause i don't just want an answer just a logical explanation. you so much! )

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
Questions

Chemistry, 22.07.2019 10:30







Mathematics, 22.07.2019 10:30

English, 22.07.2019 10:30


Mathematics, 22.07.2019 10:30


Chemistry, 22.07.2019 10:30


Mathematics, 22.07.2019 10:30

Biology, 22.07.2019 10:30


Mathematics, 22.07.2019 10:30

History, 22.07.2019 10:30

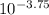 ,
, 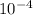 (i see this result on calculator)
(i see this result on calculator)

