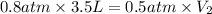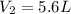Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2



Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
Acontainer of gas has a volume of 3.5 l and a pressure of 0.8 atm. assuming the temperature remains...
Questions

Arts, 06.11.2020 21:20

Computers and Technology, 06.11.2020 21:20

Arts, 06.11.2020 21:20


Mathematics, 06.11.2020 21:20


Mathematics, 06.11.2020 21:20

Mathematics, 06.11.2020 21:20


Chemistry, 06.11.2020 21:20

Biology, 06.11.2020 21:20



Spanish, 06.11.2020 21:20

Mathematics, 06.11.2020 21:20

Mathematics, 06.11.2020 21:20

Chemistry, 06.11.2020 21:20

Mathematics, 06.11.2020 21:20

Arts, 06.11.2020 21:20

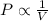
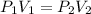
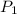 = initial pressure of gas = 0.8 atm
= initial pressure of gas = 0.8 atm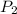 = final pressure of gas = 0.5 atm
= final pressure of gas = 0.5 atm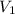 = initial volume of gas = 3.5 L
= initial volume of gas = 3.5 L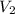 = final volume of gas = ?
= final volume of gas = ?