

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
Zn(s) + 2hcl(aq) → h2(g) + zncl2(aq) when 25.0 g of zn reacts, how many l of h2 gas are formed at st...
Questions


Mathematics, 02.12.2019 19:31

Mathematics, 02.12.2019 19:31





Computers and Technology, 02.12.2019 19:31












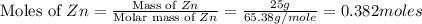
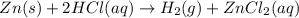
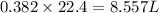 volume of hydrogen gas.
volume of hydrogen gas.

