
Fluorine (f) and bromine (br) are in the same group on the periodic table. how do atoms of these elements compare when they form bonds?
1-both a fluorine atom and a bromine atom lose one electron, and both atoms become stable.
2-a fluorine atom becomes stable by losing one electron, but a bromine atom cannot become stable by losing only one electron.
3-both a fluorine atom and a bromine atom gain one electron, and both atoms become stable.
4-a fluorine atom becomes stable by gaining one electron, but a bromine atom cannot become stable by gaining only one electron.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1
You know the right answer?
Fluorine (f) and bromine (br) are in the same group on the periodic table. how do atoms of these ele...
Questions

French, 19.03.2021 18:10

Mathematics, 19.03.2021 18:10




Mathematics, 19.03.2021 18:10



Mathematics, 19.03.2021 18:10

Chemistry, 19.03.2021 18:10





Computers and Technology, 19.03.2021 18:10


Mathematics, 19.03.2021 18:10

Health, 19.03.2021 18:10


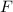 and bromine,
and bromine, 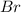 are in the same group on the periodic table that is halogen group (Group 17). The halogen group has electronic configuration as
are in the same group on the periodic table that is halogen group (Group 17). The halogen group has electronic configuration as 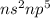 that means they are deficient of one electron to complete their octet and attain stability. So, they gain 1 electron and result in the formation of anion and form compounds by transfer of electrons.
that means they are deficient of one electron to complete their octet and attain stability. So, they gain 1 electron and result in the formation of anion and form compounds by transfer of electrons. 

