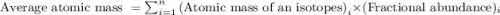Hydrogen has three isotopes with mass numbers of 1, 2, and 3 and has an average atomic mass of 1.00794 amu. this information indicates that
1. equal numbers of each isotope are present
2. more isotopes have an atomic mass of 2 or 3 than of 1
3. more isotopes have an atomic mass of 1 than of 2 or 3
4. isotopes have only an atomic mass of 1

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
The overall chemical reaction for photosynthesis isshown below: 6co2 + 6h20 → c6h12o6 + 602what mass of glucose (c6h1206) can form from71.89 g co2? (molar mass of c6h1206 = 180.18g/mol; molar mass of co2 = 44.01 g/mol)71.89 g co2=g c6h1206
Answers: 1

Chemistry, 21.06.2019 19:00
Asmall amount of a solid is added to water. the observation made after fifteen minutes is shown in the figure. which of these solids has been probably added to water? a) oil b) sand c) sugar d) wood chips
Answers: 1

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1
You know the right answer?
Hydrogen has three isotopes with mass numbers of 1, 2, and 3 and has an average atomic mass of 1.007...
Questions



Mathematics, 06.10.2019 08:00



Mathematics, 06.10.2019 08:00













Mathematics, 06.10.2019 08:00