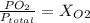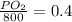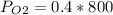Chemistry, 13.11.2019 09:31 blueflu5120
Amixture of gases with a pressure of 800.0 mm hg contains 60% nitrogen and 40% oxygen by volume. what is the partial pressure of oxygen in this mixture?
a. 140.0 mm hg
b. 320.0 mm hg
c. 373.0 mm hg
d. 480.0 mm hg

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3



Chemistry, 23.06.2019 06:50
Organisms are classified as producer or consumers according to the way they ?
Answers: 1
You know the right answer?
Amixture of gases with a pressure of 800.0 mm hg contains 60% nitrogen and 40% oxygen by volume. wha...
Questions

Physics, 16.10.2020 05:01

Physics, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01




Mathematics, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01

English, 16.10.2020 05:01

Social Studies, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01


French, 16.10.2020 05:01

French, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01



Mathematics, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01

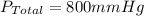
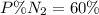
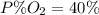
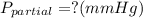
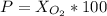
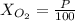
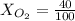
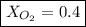
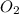 :
: