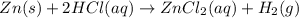Chemistry, 01.09.2019 02:00 kritalewis
The balanced chemical equation, zn (s) + 2 hcl (aq) zncl2 (aq) + h2 (g), can be expressed in words as: answer zirconium metal plus hydrogen chloride yields zirconium chloride solution and hydrogen gas xenon solid plus hydrochloric acid yields xenon chloride and hydrogen gas zinc metal plus hydrogen chloride yields zinc dichloride plus hydrogen gas zinc metal plus an aqueous solution of hydrochloric acid yields an aqueous solution of zinc chloride plus hydrogen gas

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3
You know the right answer?
The balanced chemical equation, zn (s) + 2 hcl (aq) zncl2 (aq) + h2 (g), can be expressed in words a...
Questions


Biology, 04.06.2021 02:00

English, 04.06.2021 02:00



Computers and Technology, 04.06.2021 02:00





Mathematics, 04.06.2021 02:00

Mathematics, 04.06.2021 02:00



Biology, 04.06.2021 02:00

English, 04.06.2021 02:00

Mathematics, 04.06.2021 02:00


Mathematics, 04.06.2021 02:00

Arts, 04.06.2021 02:00

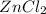 is the molecular formula of Zinc chloride
is the molecular formula of Zinc chloride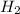 molecular formula of hydrogen gas.
molecular formula of hydrogen gas.