
Chemistry, 22.01.2020 02:31 alexamorantess
Calculate δgrxn for this equation, rounding your answer to the nearest whole number. caco3(s) → cao(s) + co2(g) δgf, caco3 = −1,128.76 kj/mol δgf, cao = −604.17 kj/mol δgf, co2 = −394.4 kj/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3


Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
Calculate δgrxn for this equation, rounding your answer to the nearest whole number. caco3(s) → cao(...
Questions


Mathematics, 04.08.2020 14:01

English, 04.08.2020 14:01

Mathematics, 04.08.2020 14:01

SAT, 04.08.2020 14:01


Biology, 04.08.2020 14:01


Mathematics, 04.08.2020 14:01

English, 04.08.2020 14:01



Mathematics, 04.08.2020 14:01


Biology, 04.08.2020 14:01




Mathematics, 04.08.2020 14:01

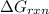 for the given reaction is 130.19kJ/mol
for the given reaction is 130.19kJ/mol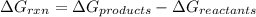
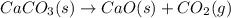
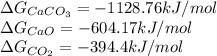
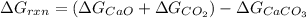
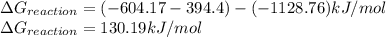
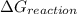 comes out to be positive, the reaction is said to be non-spontaneous reaction.
comes out to be positive, the reaction is said to be non-spontaneous reaction. 

