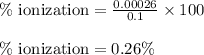Chemistry, 25.09.2019 11:00 lailabirdiemae
Calculate the percent ionization of ha in a 0.10 m solution. express your answer as a percent using two significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1

Chemistry, 23.06.2019 10:00
An uncovered pot of water lies out in the sun. which statements correctly describe what happens at the surface of the liquid water? 1. the vapor pressure remains constant regardless of the water temperature. 2. the vapor pressure is produced by water molecules that have evaporated. 3. the vapor pressure increases as the sun heats the water in the pot. 4. evaporation stops once the vapor pressure reaches a certain point. 5. evaporation and condensation both occur on the liquid’s surface.
Answers: 3
You know the right answer?
Calculate the percent ionization of ha in a 0.10 m solution. express your answer as a percent using...
Questions

Mathematics, 12.08.2020 09:01




Mathematics, 12.08.2020 09:01


Computers and Technology, 12.08.2020 09:01






Mathematics, 12.08.2020 09:01

Biology, 12.08.2020 09:01

Biology, 12.08.2020 09:01

Arts, 12.08.2020 09:01

Mathematics, 12.08.2020 09:01



Computers and Technology, 12.08.2020 09:01

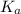 of the given acid is
of the given acid is 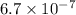
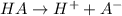
![K_a=\frac{[H^+][A^-]}{[HA]}](/tpl/images/0261/2022/66f51.png)
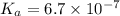
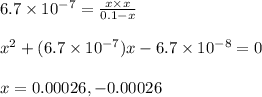
![\%\text{ ionization}=\frac{[H^+]_{eq}}{[HA]_i}\times 100](/tpl/images/0261/2022/e5a7a.png)
![[H^+]_{eq}=x=0.00026M](/tpl/images/0261/2022/1c61e.png)
![[HA]_i=0.1M](/tpl/images/0261/2022/d568f.png)