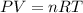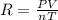Chemistry, 01.09.2019 20:50 tugordochulo1
If the pressure, volume, and the number of moles of a gas are known, which is needed to calculate the universal gas constant from the ideal gas law?
the temperature of the gas
the molar volume of the gas
the molar mass of the gas
the partial pressure of the gas

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2
You know the right answer?
If the pressure, volume, and the number of moles of a gas are known, which is needed to calculate th...
Questions

















Mathematics, 02.11.2019 06:31


Mathematics, 02.11.2019 06:31