
Chemistry, 31.12.2019 14:31 rosemarybooker
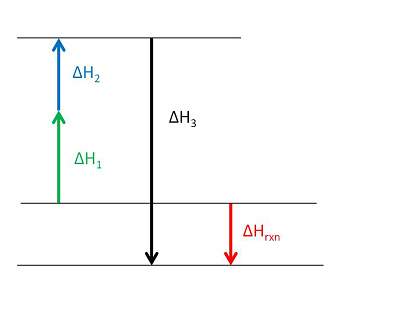
Consider the following enthalpy diagram and enthalpies of intermediate and overall chemical reactions.
which of the following statements is true?
the overall chemical reaction is exothermic.
there are two intermediate reactions in this system.
the third intermediate reaction is endothermic.
the longest arrow represents the overall chemical reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1
You know the right answer?
Consider the following enthalpy diagram and enthalpies of intermediate and overall chemical reaction...
Questions



Mathematics, 12.08.2021 14:00

Biology, 12.08.2021 14:00

Mathematics, 12.08.2021 14:00




English, 12.08.2021 14:00

Social Studies, 12.08.2021 14:00

Mathematics, 12.08.2021 14:00



English, 12.08.2021 14:00

English, 12.08.2021 14:00

Arts, 12.08.2021 14:00






