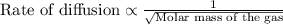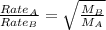Chemistry, 13.11.2019 13:31 zaniyastubbs9
Asample of gas a has a molar mass of 4 grams while a sample of gas b has a molar mass of 16 grams. which statement holds true?
both gas a and gas b diffuse at the same speed.
gas a effuses faster than gas b.
gas b effuses faster than gas a.
the molar masses of gas a and gas b are not related to effusion.
the molar mass is directly proportional to the rate of effusion.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
Asample of gas a has a molar mass of 4 grams while a sample of gas b has a molar mass of 16 grams. w...
Questions

Chemistry, 04.02.2020 05:54




Advanced Placement (AP), 04.02.2020 05:54

Mathematics, 04.02.2020 05:54

Mathematics, 04.02.2020 05:54

History, 04.02.2020 05:54


Physics, 04.02.2020 05:54


Mathematics, 04.02.2020 05:54





Social Studies, 04.02.2020 05:54



Mathematics, 04.02.2020 05:54