
Chemistry, 06.10.2019 00:00 sirdre1982
Calculate the molarity of a solution prepared by dissolving 6.80 grams of agno3 in enough water to make 2.50 liters of solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

You know the right answer?
Calculate the molarity of a solution prepared by dissolving 6.80 grams of agno3 in enough water to m...
Questions

Mathematics, 08.01.2020 01:31


Mathematics, 08.01.2020 01:31


Mathematics, 08.01.2020 01:31

Mathematics, 08.01.2020 01:31


History, 08.01.2020 01:31


Geography, 08.01.2020 01:31







Business, 08.01.2020 01:31



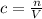
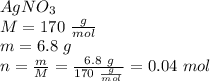
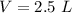 .
.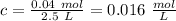 .
.

