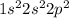Question
a carbon atom is able to form four single covalent bonds because it
a) has fou...

Chemistry, 02.01.2020 11:31 23rwilliamson
Question
a carbon atom is able to form four single covalent bonds because it
a) has four electrons and four protons.
b) has four valence electrons in its outermost energy level.
c) forms a polyatomic ion with a -4 charge.
d) forms a more stable isotope when it gains four more electrons.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1
You know the right answer?
Questions




Mathematics, 18.03.2021 01:50


History, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50

English, 18.03.2021 01:50


Mathematics, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50






Mathematics, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50

English, 18.03.2021 01:50