
Chemistry, 28.09.2019 19:10 ryleerose255
If 3.0 moles of a and 4.0 moles of b react according to the hypothetical reaction below, how many moles of the excess reactant will be left over at the end of the reaction? a + 2b → ab2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1
You know the right answer?
If 3.0 moles of a and 4.0 moles of b react according to the hypothetical reaction below, how many mo...
Questions


History, 21.10.2020 14:01



Mathematics, 21.10.2020 14:01

Mathematics, 21.10.2020 14:01


Mathematics, 21.10.2020 14:01

English, 21.10.2020 14:01

Mathematics, 21.10.2020 14:01


Health, 21.10.2020 14:01


Social Studies, 21.10.2020 14:01

Mathematics, 21.10.2020 14:01


Mathematics, 21.10.2020 14:01

English, 21.10.2020 14:01

Mathematics, 21.10.2020 14:01

Mathematics, 21.10.2020 14:01

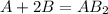
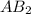
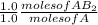 = 3.0 moles of
= 3.0 moles of 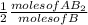 = 2.0 moles of
= 2.0 moles of 

