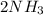Chemistry, 30.09.2019 00:30 markaylarowland8
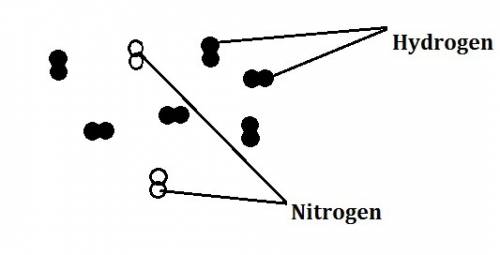
In the haber process, nitrogen (n2) and hydrogen (h2) are directly combined to form ammonia (nh3). which illustration contains the stoichiometric quantities of the reactants for this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1


Chemistry, 23.06.2019 08:40
The half-life of a certain element is 100 days. how many half-lives will it be before only one eighth of this elementremains?
Answers: 1
You know the right answer?
In the haber process, nitrogen (n2) and hydrogen (h2) are directly combined to form ammonia (nh3). w...
Questions










History, 05.03.2020 07:46

Computers and Technology, 05.03.2020 07:46



Mathematics, 05.03.2020 07:46






Physics, 05.03.2020 07:48

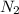 +
+ 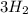 =
=