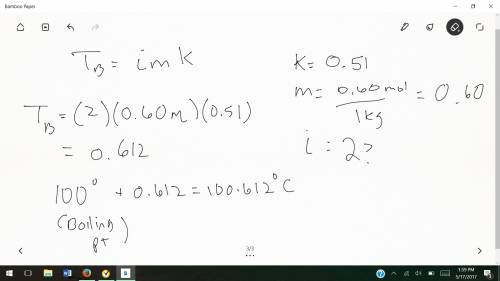
Chemistry, 09.10.2019 19:30 skylar1315
Determine the boiling point of a solution made by dissolving 0.60 mol k2so4 in 1.0 kg water. water has a boiling point elevation constant of 0.51°c•kg/mol. what is the boiling point of this solution?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
Determine the boiling point of a solution made by dissolving 0.60 mol k2so4 in 1.0 kg water. water h...
Questions


Mathematics, 22.03.2021 21:30







Mathematics, 22.03.2021 21:30


Chemistry, 22.03.2021 21:30



Biology, 22.03.2021 21:30

Chemistry, 22.03.2021 21:30