

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
which two values for ∆g and e0cell correctly indicate a spontaneous reaction? a) ∆g = -89 kj, e0cel...
Questions

Arts, 20.01.2021 16:30

Chemistry, 20.01.2021 16:30



English, 20.01.2021 16:30

Mathematics, 20.01.2021 16:30

English, 20.01.2021 16:30

Mathematics, 20.01.2021 16:30

Mathematics, 20.01.2021 16:30

Mathematics, 20.01.2021 16:30

Biology, 20.01.2021 16:30

History, 20.01.2021 16:30

Mathematics, 20.01.2021 16:30

Mathematics, 20.01.2021 16:30

Mathematics, 20.01.2021 16:30





Physics, 20.01.2021 16:30

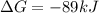 and
and 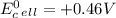
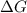 stands for Gibbs free energy equation. A reaction is spontaneous if
stands for Gibbs free energy equation. A reaction is spontaneous if 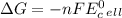
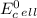 is standard cell potential.
is standard cell potential. 

