

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Consider this equilibrium constant expression: k= (h2s)(oh-)/(hs-) what does this expression repres...
Questions




Mathematics, 08.10.2021 05:00





Mathematics, 08.10.2021 05:00


Mathematics, 08.10.2021 05:00


Mathematics, 08.10.2021 05:00

Social Studies, 08.10.2021 05:00

Mathematics, 08.10.2021 05:00

Mathematics, 08.10.2021 05:00


History, 08.10.2021 05:00

Business, 08.10.2021 05:00

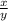 ), the solution is Kb for HS-
), the solution is Kb for HS-


