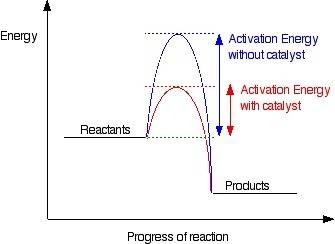
How do catalysts increase the rate of a chemical reaction?
they lower the activation energy.<...

Chemistry, 23.01.2020 10:31 christianmason9423
How do catalysts increase the rate of a chemical reaction?
they lower the activation energy.
they lower the collision energy.
they increase the collision energy.
they increase the temperature.
they increase the concentration of reactants.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2


Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
Questions

Mathematics, 13.04.2020 23:31



History, 13.04.2020 23:31

Mathematics, 13.04.2020 23:31

Mathematics, 13.04.2020 23:31







Mathematics, 13.04.2020 23:32

Biology, 13.04.2020 23:32





Mathematics, 13.04.2020 23:32