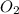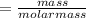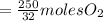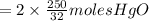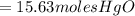Mercury(ii) oxide (hgo) decomposes to form mercury (hg) and oxygen (o2). the balanced chemical equation is shown below.
2hgo 2hg + o2
the molar mass of hgo is 216.59 g/mol. the molar mass of o2 is 32.00 g/mol. how many moles of hgo are needed to produce 250.0 g of o2?
3.906
7.813
15.63
73.87

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1
You know the right answer?
Mercury(ii) oxide (hgo) decomposes to form mercury (hg) and oxygen (o2). the balanced chemical equat...
Questions


Mathematics, 27.11.2021 03:30

Mathematics, 27.11.2021 03:30





SAT, 27.11.2021 03:40

Mathematics, 27.11.2021 03:40


Social Studies, 27.11.2021 03:40