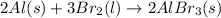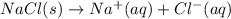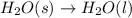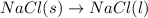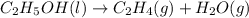Chemistry, 29.09.2019 05:30 rissacoob7862
Which of the following processes would you expect to have a negative value for entropy? nacl(s) na+(aq) + cl- (aq) h2o(s) h2o(l) nacl(s) nacl(l) 2 al(s) + 3br2(l) 2albr3(s) c2h5oh(l) c2h4(g) + h2o(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
Which of the following processes would you expect to have a negative value for entropy? nacl(s) na+...
Questions




Mathematics, 22.09.2019 13:30

Business, 22.09.2019 13:30



Geography, 22.09.2019 13:30



Biology, 22.09.2019 13:30

History, 22.09.2019 13:30

Biology, 22.09.2019 13:30

Biology, 22.09.2019 13:30


History, 22.09.2019 13:30

Social Studies, 22.09.2019 13:30

Mathematics, 22.09.2019 13:30

Mathematics, 22.09.2019 13:30