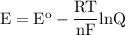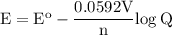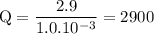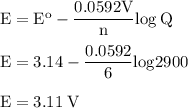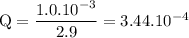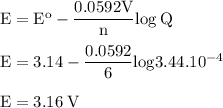Chemistry, 28.09.2019 01:40 hdwoody2002
Answer this question asap. this is on a homework assignment that is due you in advance! a voltaic cell employs the following redox reaction: 2fe3+(aq)+3mg(s)→2fe(s)+3mg2+(aq) calculate the cell potential at 25 ∘c under each of the following conditions.
a. find ecell at standard conditions
b. when [fe3+]= 1.0×10−3 m ; [mg2+]= 2.90 m find ecell
c. when [fe3+]=2.90m; [mg2+]=1.0*10^-3 m find e cell

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2
You know the right answer?
Answer this question asap. this is on a homework assignment that is due you in advance! a voltaic...
Questions





Mathematics, 12.04.2021 01:20


Mathematics, 12.04.2021 01:20

Mathematics, 12.04.2021 01:20

Mathematics, 12.04.2021 01:20

Computers and Technology, 12.04.2021 01:20

Mathematics, 12.04.2021 01:20




Mathematics, 12.04.2021 01:20





Mathematics, 12.04.2021 01:20