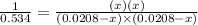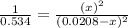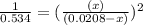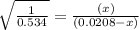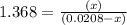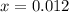Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1


Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1
You know the right answer?
For the reaction given below at 700°c, kc = 0.534. h2(g + co2(g h2o(g + co(g calculate the number of...
Questions


History, 16.02.2020 06:50

Mathematics, 16.02.2020 06:50

Mathematics, 16.02.2020 06:50


Social Studies, 16.02.2020 06:51

Business, 16.02.2020 06:51

Social Studies, 16.02.2020 06:51

Mathematics, 16.02.2020 06:51

Mathematics, 16.02.2020 06:52





Health, 16.02.2020 06:53


English, 16.02.2020 06:53

Mathematics, 16.02.2020 06:53


English, 16.02.2020 06:53

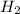 at equilibrium is 0.012 M
at equilibrium is 0.012 M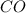 and
and 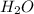 .
.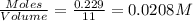
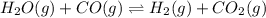
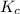 will be,
will be,![K_c=\frac{[H_2][CO_2]}{[H_2O][CO]}](/tpl/images/0263/7107/ded1c.png)
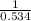 (for reverse reaction).
(for reverse reaction).