Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 23.06.2019 07:50
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1

Chemistry, 23.06.2019 09:10
A155.0 9 piece of copper at 182 °c is dropped into 2500 g of water at 23.9 °c. (the specific heat of copper is 0.385 1/9°c.) calculate the final temperature of the mixture. (assume no heat loss to the surroundings.)
Answers: 2
You know the right answer?
A150 ml sample of hydrochloric acid (hcl) completely reacted with 60.0 ml of a 0.100 m naoh solution...
Questions


Mathematics, 27.09.2020 21:01

Mathematics, 27.09.2020 21:01



History, 27.09.2020 21:01

Mathematics, 27.09.2020 21:01

Mathematics, 27.09.2020 21:01

Mathematics, 27.09.2020 21:01



English, 27.09.2020 21:01

Mathematics, 27.09.2020 21:01

Advanced Placement (AP), 27.09.2020 21:01

Mathematics, 27.09.2020 21:01



History, 27.09.2020 21:01



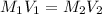
 = molarity of NaOH solution = 0.100 M
= molarity of NaOH solution = 0.100 M
 = volume of NaOH solution = 60.0 ml
= volume of NaOH solution = 60.0 ml = molarity of HCl solution = ?
= molarity of HCl solution = ?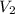 = volume of HCl solution = 150 ml
= volume of HCl solution = 150 ml