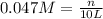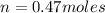Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2

Chemistry, 24.06.2019 01:00
Chemical hand warmers popular with skiers and snowboarders produce heat when they are removed from their airtight plastic wrappers. they utilize the oxidation of iron to form iron(iii) oxide according to the reaction:
Answers: 2

You know the right answer?
Pcl5 => pcl3+cl2
the no of moles of cl2 produced will be if one mole of pcl5 is heated 25...
the no of moles of cl2 produced will be if one mole of pcl5 is heated 25...
Questions


French, 11.01.2021 20:30

Mathematics, 11.01.2021 20:30

Biology, 11.01.2021 20:30



History, 11.01.2021 20:30




English, 11.01.2021 20:30


Computers and Technology, 11.01.2021 20:30




Mathematics, 11.01.2021 20:30



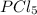 = 1 mole
= 1 mole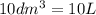
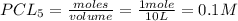
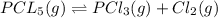
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0394/8934/73fe0.png)
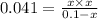
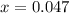
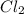 produced is 0.047 M
produced is 0.047 M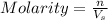
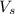 = volume of solution in L
= volume of solution in L