
Chemistry, 18.09.2019 02:00 hchxxhBfncndnd9319
The haber process can be used to produce ammonia (nh3) from hydrogen gas (h2) and nitrogen gas (n2). the balanced equation for this process is shown below.
3h2 + n2 mc025-1.jpg 2nh3
the molar mass of nh3 is 17.03 g/mol. the molar mass of h2 is 2.0158 g/mol. in a particular reaction, 0.575 g of nh3 forms. what is the mass, in grams, of h2 that must have reacted, to the correct number of significant figures?
0.1
0.102
0.10209
0.1021

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
The haber process can be used to produce ammonia (nh3) from hydrogen gas (h2) and nitrogen gas (n2)....
Questions

Biology, 03.08.2020 14:01

Chemistry, 03.08.2020 14:01

English, 03.08.2020 14:01


History, 03.08.2020 14:01


English, 03.08.2020 14:01


Mathematics, 03.08.2020 14:01


Biology, 03.08.2020 14:01


Computers and Technology, 03.08.2020 14:01




Mathematics, 03.08.2020 14:01


Computers and Technology, 03.08.2020 14:01

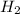 in grams is 0.102g.
in grams is 0.102g.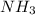 = 17.03 g/mole
= 17.03 g/mole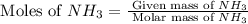 =
= 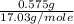 = 0.0337 moles
= 0.0337 moles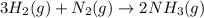
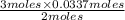 moles of
moles of 


