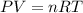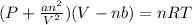a. at all temperatures and pressures

under what conditions do most gases behave ideally?
a. at all temperatures and pressures
b. at high temperatures and pressure above 10 atm
c. at extremely low temperature and low pressure
d. at low temperature and high pressure
e. at high temperature and low pressure

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
You know the right answer?
under what conditions do most gases behave ideally?
a. at all temperatures and pressures
a. at all temperatures and pressures
Questions

Chemistry, 01.02.2020 23:45

Social Studies, 01.02.2020 23:45





History, 01.02.2020 23:45

Mathematics, 01.02.2020 23:45



Mathematics, 01.02.2020 23:45

History, 01.02.2020 23:45


Mathematics, 01.02.2020 23:45

Geography, 01.02.2020 23:45

Health, 01.02.2020 23:45

English, 01.02.2020 23:45


Mathematics, 01.02.2020 23:45