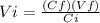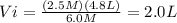Chemistry, 04.01.2020 15:31 llamasking
Alaboratory experiment requires 4.8 l of a 2.5 m solution of sulfuric acid (h2so4), but the only available h2so4 is a 6.0 m stock solution. how could you prepare the solution needed for the lab experiment? show all the work used to find your answer.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
Alaboratory experiment requires 4.8 l of a 2.5 m solution of sulfuric acid (h2so4), but the only ava...
Questions

English, 22.04.2021 22:50

Geography, 22.04.2021 22:50

Mathematics, 22.04.2021 22:50

Mathematics, 22.04.2021 22:50



Mathematics, 22.04.2021 22:50

Mathematics, 22.04.2021 22:50


History, 22.04.2021 22:50

Mathematics, 22.04.2021 22:50




Mathematics, 22.04.2021 22:50



Mathematics, 22.04.2021 22:50