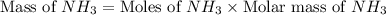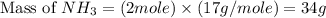Chemistry, 28.08.2019 15:00 CHENDESHEN
When 28 g of nitrogen and 6 g of hydrogen react, 34 g of ammonia are produced. if 100 g of nitrogen react with 6 g of hydrogen, how much ammonia will be produced?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
When 28 g of nitrogen and 6 g of hydrogen react, 34 g of ammonia are produced. if 100 g of nitrogen...
Questions

Arts, 01.10.2019 01:00

Physics, 01.10.2019 01:00

Biology, 01.10.2019 01:00

Geography, 01.10.2019 01:00


Biology, 01.10.2019 01:00



English, 01.10.2019 01:00


English, 01.10.2019 01:00


Health, 01.10.2019 01:00

History, 01.10.2019 01:00

History, 01.10.2019 01:00



Biology, 01.10.2019 01:00


Biology, 01.10.2019 01:00

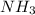 produced will be, 34 grams.
produced will be, 34 grams.
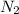 = 100 g
= 100 g
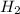 = 6 g
= 6 g
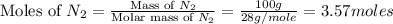
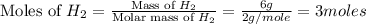
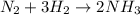
 moles of
moles of