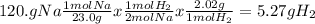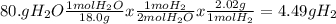Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
You know the right answer?
For the reaction represented by the equation 2na + 2h2o ® 2naoh + h2, how many grams of hydrogen are...
Questions






Mathematics, 02.12.2019 02:31


Mathematics, 02.12.2019 02:31

Social Studies, 02.12.2019 02:31

Mathematics, 02.12.2019 02:31




History, 02.12.2019 02:31



English, 02.12.2019 02:31


Mathematics, 02.12.2019 02:31