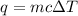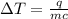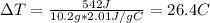The specific heat of a substance is 2.01 j/g·°
c. if given 10.3 grams of the substance and if...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1


Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
Questions


Mathematics, 25.06.2020 07:01

Mathematics, 25.06.2020 07:01


Mathematics, 25.06.2020 07:01


Biology, 25.06.2020 07:01


Mathematics, 25.06.2020 07:01



Mathematics, 25.06.2020 07:01

History, 25.06.2020 07:01

Mathematics, 25.06.2020 07:01



Mathematics, 25.06.2020 07:01

Mathematics, 25.06.2020 07:01

Mathematics, 25.06.2020 07:01