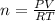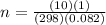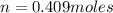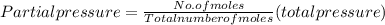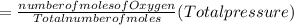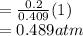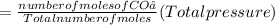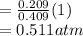Chemistry, 13.10.2019 23:30 jasminelynn135owmyj1
A10 liter flask at 298 k contains a gaseous mixture of o2 and co2 at 1 atmosphere. which statement is true for the partial pressures of o2 and co2 if 0.2 mole of o2 is present in the flask? (given the universal gas constant r = 0.082 l∙atm/k∙mol)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

You know the right answer?
A10 liter flask at 298 k contains a gaseous mixture of o2 and co2 at 1 atmosphere. which statement i...
Questions


Mathematics, 21.10.2021 03:50

English, 21.10.2021 03:50

English, 21.10.2021 04:00


Physics, 21.10.2021 04:00





Mathematics, 21.10.2021 04:00


Mathematics, 21.10.2021 04:00



Mathematics, 21.10.2021 04:00

Social Studies, 21.10.2021 04:00

Biology, 21.10.2021 04:00

Biology, 21.10.2021 04:00