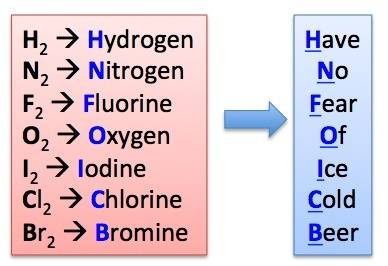
Chemistry, 10.10.2019 18:50 drainy0uandthefish
Which of the following elements exists as a diatomic molecule? argon, hydrogen, boron, sulfur

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 07:30
How do you interpret a chromagram for what mixtures contain?
Answers: 1

Chemistry, 23.06.2019 13:30
Explain the impact that changing the temperature has on a system in a state of dynamic equilibrium. what will happen when the temperature of an exothermic reaction mixture at equilibrium is increased?
Answers: 3
You know the right answer?
Which of the following elements exists as a diatomic molecule? argon, hydrogen, boron, sulfur...
Questions


Biology, 17.06.2021 19:10










Arts, 17.06.2021 19:10

Mathematics, 17.06.2021 19:10


English, 17.06.2021 19:10


Mathematics, 17.06.2021 19:10

Mathematics, 17.06.2021 19:10