
Chemistry, 17.10.2019 03:00 Foxfire5109
How many liters of methane gas (ch4) need to be combusted to produce 8.5 liters of water vapor, if all measurements are taken at the same temperature and pressure? show all of the work used to solve this problem. ch4 (g) + 2o2 (g) yields co2 (g) + 2h2o (g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

You know the right answer?
How many liters of methane gas (ch4) need to be combusted to produce 8.5 liters of water vapor, if a...
Questions


Mathematics, 31.01.2020 03:02

Mathematics, 31.01.2020 03:02



English, 31.01.2020 03:02

Mathematics, 31.01.2020 03:02

Mathematics, 31.01.2020 03:02


Biology, 31.01.2020 03:02

English, 31.01.2020 03:02

Social Studies, 31.01.2020 03:02


Mathematics, 31.01.2020 03:02

Mathematics, 31.01.2020 03:02

Chemistry, 31.01.2020 03:03

Mathematics, 31.01.2020 03:03

History, 31.01.2020 03:03


Mathematics, 31.01.2020 03:03

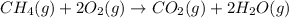
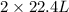 volume of water vapor produced from 22.4 L volume of methane gas
volume of water vapor produced from 22.4 L volume of methane gas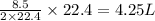 volume of methane gas
volume of methane gas

