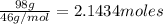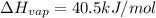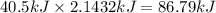Chemistry, 02.10.2019 10:50 moniquejg1800
How much energy is required to vaporize 98.6 g of ethanol (c2h5oh) at its boiling point, if its δhvap is 40.5 kj/mol? how much energy is required to vaporize 98.6 g of ethanol (c2h5oh) at its boiling point, if its δhvap is 40.5 kj/mol? 52.8 kj 11.5 kj 86.7 kj 39.9 kj 18.9 kj

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

You know the right answer?
How much energy is required to vaporize 98.6 g of ethanol (c2h5oh) at its boiling point, if its δhva...
Questions

History, 15.02.2021 01:00



Mathematics, 15.02.2021 01:00



Mathematics, 15.02.2021 01:00

Mathematics, 15.02.2021 01:00

History, 15.02.2021 01:00

Chemistry, 15.02.2021 01:00

Physics, 15.02.2021 01:00

Chemistry, 15.02.2021 01:00

Mathematics, 15.02.2021 01:00

History, 15.02.2021 01:00




Chemistry, 15.02.2021 01:00