
Chemistry, 06.10.2019 12:20 destinywiggins75
Which is a characteristic of weak acids and weak bases?
a. ka and kb values of weak acids and weak bases are small.
b. forward reactions of weak acids and weak bases are favored.
c. dissociation of weak acids and weak bases is nearly complete.
d. many h+ or oh– ions are present in their solution.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
Which is a characteristic of weak acids and weak bases?
a. ka and kb values of weak acids and...
a. ka and kb values of weak acids and...
Questions

Arts, 19.05.2021 01:00

Mathematics, 19.05.2021 01:00

Mathematics, 19.05.2021 01:00

Mathematics, 19.05.2021 01:00

Mathematics, 19.05.2021 01:00

Mathematics, 19.05.2021 01:00



Mathematics, 19.05.2021 01:00



Mathematics, 19.05.2021 01:00


German, 19.05.2021 01:00



Mathematics, 19.05.2021 01:00



Mathematics, 19.05.2021 01:00

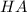 ⇔
⇔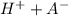
![K_{a}=\frac {[H^{+}][A^{-}]}{[HA]}](/tpl/images/0293/5761/dc648.png)
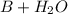 ⇔
⇔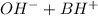
![K_{b}=\frac {[OH^{-}][BH^{+}]}{[B]}](/tpl/images/0293/5761/e6d15.png)



