
Chemistry, 24.10.2019 18:43 kenyakids405
In the following combustion reaction of acetylene (c2h2), how many liters of co2 will be produced if 60 liters of o2 is used, given that both gases are at stp? 2c2h2+5o2 2h2o+4co2 the volume of one mole of gas at stp is 22.4 liters. 48 liters 0.02 liters 240 liters 300 liters nextreset

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
You know the right answer?
In the following combustion reaction of acetylene (c2h2), how many liters of co2 will be produced if...
Questions

Health, 13.01.2021 20:20

Advanced Placement (AP), 13.01.2021 20:20




Mathematics, 13.01.2021 20:20


English, 13.01.2021 20:20

Mathematics, 13.01.2021 20:20



Mathematics, 13.01.2021 20:30

History, 13.01.2021 20:30

Mathematics, 13.01.2021 20:30

Mathematics, 13.01.2021 20:30

Social Studies, 13.01.2021 20:30


Social Studies, 13.01.2021 20:30

Mathematics, 13.01.2021 20:30

History, 13.01.2021 20:30

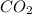 produced will be, 48 liters.
produced will be, 48 liters.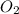 = 60 L
= 60 L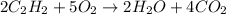
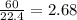 mole of
mole of 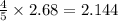 moles of
moles of 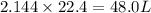 volume of
volume of 

