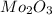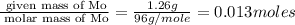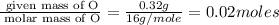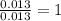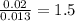Acompound that is composed of molybdenum (mo) and oxygen (o) was produced in a lab by heating molybdenum over a bunsen burner. the following data was collected:
mass of crucible: 38.26 g
mass of crucible and molybdenum: 39.52 g
mass of crucible and molybdenum oxide: 39.84 g
solve for the empirical formula of the compound, showing your calculations.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1

Chemistry, 23.06.2019 05:30
Find the midpoint of a segment with endpoints of 4-3i and -2+7i
Answers: 2
You know the right answer?
Acompound that is composed of molybdenum (mo) and oxygen (o) was produced in a lab by heating molybd...
Questions

English, 14.05.2021 23:40

Mathematics, 14.05.2021 23:40

Mathematics, 14.05.2021 23:40

Mathematics, 14.05.2021 23:40




Arts, 14.05.2021 23:40

Mathematics, 14.05.2021 23:40




Mathematics, 14.05.2021 23:40




Social Studies, 14.05.2021 23:40