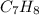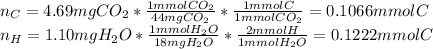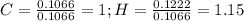Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3
You know the right answer?
Combustion analysis of toluene, a common organic solvent, gives 4.69 mg of co2 and 1.10 mg of h2o. i...
Questions


Mathematics, 02.07.2019 17:20

English, 02.07.2019 17:20


History, 02.07.2019 17:20

Mathematics, 02.07.2019 17:20

Mathematics, 02.07.2019 17:20


History, 02.07.2019 17:20

Mathematics, 02.07.2019 17:20




Mathematics, 02.07.2019 17:20

Biology, 02.07.2019 17:20