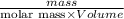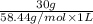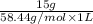Chemistry, 23.09.2019 07:20 msitez2442
Which of the following statements is true of an aqueous solution of sodium chloride? a]one that has 30.0 grams of nacl dissolved in 100 grams of water is more concentrated than one that has 15.0 grams of nacl dissolved in 100 grams of water. b]one that has 30.0 grams of nacl dissolved in 100 grams of water is more dilute than one that has 15.0 grams of nacl dissolved in 100 grams of water. c[one that has 30.0 grams of nacl dissolved in 100 grams of water would have the same molarity as one that has 15.0 grams of nacl dissolved in 100 grams of water. d]one that has 30.0 grams of nacl dissolved in 100 grams of water has less solute than one that has 15.0 grams of nacl dissolved in 100 grams of water.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3

Chemistry, 23.06.2019 11:00
Which of the following reactions represents an exothermic reaction? nh3(g) + 12.0 kcal ½n2(g) + 3/2 h2(g) ch4 + 2o2 co2 + 2h2o + 212,800 cal c + 2s cs2, h = 27,550 cal c(graphite) c(diamond), h = 0.45 kcal 2h2o 2h2 + o2, h = +58 kcal
Answers: 1
You know the right answer?
Which of the following statements is true of an aqueous solution of sodium chloride? a]one that has...
Questions


Computers and Technology, 19.10.2019 05:00

Mathematics, 19.10.2019 05:00


Biology, 19.10.2019 05:00

Physics, 19.10.2019 05:00


Chemistry, 19.10.2019 05:00

English, 19.10.2019 05:00

English, 19.10.2019 05:00


Mathematics, 19.10.2019 05:00

Mathematics, 19.10.2019 05:00