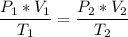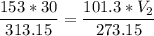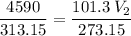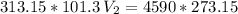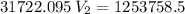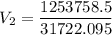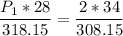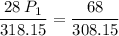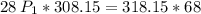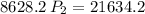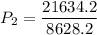Chemistry, 05.10.2019 09:01 zhellyyyyy
The volume of a gas-filled balloon is 30.0 l at 40 °c and 153 kpa pressure. what volume will the balloon have at standard temperature and pressure (273.15 k and 101.3 kpa)?
a. 17.3 l
b. 23.7 l
c. 39.5 l
d. 51.9 l
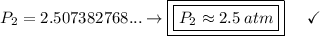
a gas that has a volume of 28 liters, a temperature of 45 °c, and an unknown pressure, has its volume increased to 34 liters and its temperature decreased to 35 °c. if i measure the pressure after the change to be 2.0 atm, what was the original pressure of the gas?
a. 1.5 atm
b. 1.7 atm
c. 2.8 atm
d. 2.5 atm

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2


Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2
You know the right answer?
The volume of a gas-filled balloon is 30.0 l at 40 °c and 153 kpa pressure. what volume will the bal...
Questions





English, 04.03.2021 14:30

Mathematics, 04.03.2021 14:30

Social Studies, 04.03.2021 14:30



Mathematics, 04.03.2021 14:30

Mathematics, 04.03.2021 14:30



Mathematics, 04.03.2021 14:30

Mathematics, 04.03.2021 14:30


Physics, 04.03.2021 14:30

History, 04.03.2021 14:30

French, 04.03.2021 14:30