3al + 3nh4clo4 → al2o3 + alcl3 + 3no + 6h2o
how many moles of alcl3 are produced?...

Chemistry, 13.10.2019 00:30 rachelsweeney10
3al + 3nh4clo4 → al2o3 + alcl3 + 3no + 6h2o
how many moles of alcl3 are produced?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

You know the right answer?
Questions



History, 24.08.2019 23:30







Mathematics, 24.08.2019 23:30


Chemistry, 24.08.2019 23:30


History, 24.08.2019 23:30

Mathematics, 24.08.2019 23:30



Business, 24.08.2019 23:30


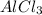 will be produced from this reaction.
will be produced from this reaction.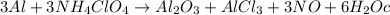
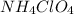 , 1 mole of
, 1 mole of 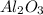 is formed.
is formed.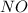 is formed.
is formed.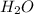 is formed.
is formed.

