Chemistry, 26.09.2019 22:40 kraigstlistt
At constant volume, the heat of combustion of a particular compound, compound a, is –3568.0 kj/mol. when 1.411 g of compound a (molar mass = 115.27 g/mol) was burned in a bomb calorimeter, the temperature of the calorimeter (including its contents) rose by 3.379 °
c. using this data, what is the heat capacity (calorimeter constant) of the calorimeter?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
In this reaction n2o4(g)→2no2(g) what changes in color would you expect as pressure is increased at a constant temperature
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
At constant volume, the heat of combustion of a particular compound, compound a, is –3568.0 kj/mol....
Questions

Biology, 23.09.2019 04:30


Mathematics, 23.09.2019 04:30


Physics, 23.09.2019 04:30



English, 23.09.2019 04:30

Chemistry, 23.09.2019 04:30

Mathematics, 23.09.2019 04:30

Business, 23.09.2019 04:30

Advanced Placement (AP), 23.09.2019 04:30

Mathematics, 23.09.2019 04:30

Computers and Technology, 23.09.2019 04:30



Social Studies, 23.09.2019 04:30


History, 23.09.2019 04:30

History, 23.09.2019 04:30

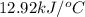
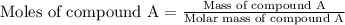

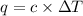
 = change in temperature =
= change in temperature =