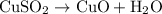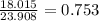Chemistry, 16.09.2019 18:40 juicemankinnie95
In the decomposition reaction, 1 mole of water (mw = 18.015 g/mol) was produced for every mole of cuo (mw = 79.545 g/mol) produced. given that 3.327 g of cuo was produced during the reaction, how many grams of water were released as water vapor? (number of moles = mass (g) / molar mass (g/ choose the closest answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1


Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
In the decomposition reaction, 1 mole of water (mw = 18.015 g/mol) was produced for every mole of cu...
Questions

Mathematics, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00




Mathematics, 17.11.2020 01:00


Chemistry, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00


Mathematics, 17.11.2020 01:00