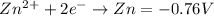Chemistry, 16.10.2019 12:30 alisaharnauth
Which half reaction is most easily oxidized?
ag+ + e− → ag = 0.80 v
br2 + 2e− → 2br− = 1.07 v
fe3+ + e− → fe2+ = 0.77 v
zn2+ + 2e− → zn = –0.76 v

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 13:10
The last few miles of the marathon are the most difficult for heather, her hair plastered to her head, sweat clinging to her arms, and her legs already feeling as if they had nothing left, just dead weight. after grabbing a cup of ice water, she feels the ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. in these last few miles, the breeze kicks up and she finally feels some coolness against her skin. drips of sweat, once clinging to her forehead, now spill down, and heather feels more pain as the sweat flows into her eyes.which of the following is the most likely reason why the ice struck heather’s nose when she took a drink? a) water can function as a solvent. b) water can store large amounts of heat. c) water can moderate temperatures through evaporative cooling. d) the density of water decreases when it freezes. e) water has a cohesive nature.sweat remained on heather’s forehead and arms because of the a) high salt content of sweat b) cohesive nature of water c) ability of water to moderate heat d) high evaporative cooling effect of water e) ability of water to act as a solvent
Answers: 1
You know the right answer?
Which half reaction is most easily oxidized?
ag+ + e− → ag = 0.80 v
br2 + 2...
ag+ + e− → ag = 0.80 v
br2 + 2...
Questions

Health, 22.02.2020 20:19


Mathematics, 22.02.2020 20:19


English, 22.02.2020 20:19

Mathematics, 22.02.2020 20:20


Mathematics, 22.02.2020 20:20


Mathematics, 22.02.2020 20:20

Mathematics, 22.02.2020 20:20

Mathematics, 22.02.2020 20:20

Mathematics, 22.02.2020 20:20

World Languages, 22.02.2020 20:20

Social Studies, 22.02.2020 20:20

English, 22.02.2020 20:20

English, 22.02.2020 20:20

Advanced Placement (AP), 22.02.2020 20:20

Mathematics, 22.02.2020 20:20