Chemistry, 27.10.2019 14:43 luckilyalexa
Phosphorus trichloride, pcl3, decomposes to form elemental phosphorus and chlorine. the equation is: 4pcl3 → p4 + 6cl2. balance the equation and determine the mass in grams of chlorine that would be formed if 25 grams of pcl3 (molecular mass = 137.32 g/mol) decompose. grams of cl2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 17:30
98 points you will be galileo perform the experiment to determine if objects with different mass fall at the same, or different, rates in the air and in a vacuum. before you conduct your experiment, you need to form a hypothesis. a hypothesis is a prediction of what you think will happen in the experiment. the hypothesis is a statement that describes “if” a certain set of circumstances are present “then” there will be a specific result that will occur. record your hypothesis here: record the results from step one of the experiment (dropping the objects in the air): first trial: second trial: third trial: record the results from step two of the experiment (dropping the objects in a vacuum): first trial: second trial: third trial: did the experiment support your hypothesis? using the data from your experiment, describe why you believe your hypothesis was either proven or disproven. what forces were acting on the objects dropped in the air? what force was acting on the objects dropped in the vacuum? part two: comparing forces choose two forces and compare and contrast these forces. you must provide two ways that they are alike and two ways that they are different. you may make a list, write in paragraph form, or make a chart. choose two forces and compare and contrast these forces. these must be different forces than used in the prior question. provide two ways that they are similar and two ways that they are different. you may make a list, write it out, or make a chart.
Answers: 3

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
Phosphorus trichloride, pcl3, decomposes to form elemental phosphorus and chlorine. the equation is:...
Questions

History, 28.05.2021 19:50


Mathematics, 28.05.2021 19:50

Mathematics, 28.05.2021 19:50

Mathematics, 28.05.2021 19:50


Physics, 28.05.2021 19:50


Mathematics, 28.05.2021 19:50




Chemistry, 28.05.2021 19:50




Spanish, 28.05.2021 19:50



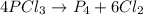
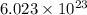 of particles.
of particles.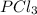 decompose to give 6 moles of
decompose to give 6 moles of 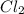
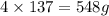 of
of 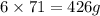 of
of 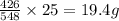 of
of