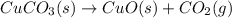Chemistry, 31.08.2019 12:20 donmak3833
When heated, solid copper(ii) carbonate decomposes to solid copper(ii) oxide and carbon dioxide gas. give the balanced chemical equation (including phases) that describes this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1
You know the right answer?
When heated, solid copper(ii) carbonate decomposes to solid copper(ii) oxide and carbon dioxide gas....
Questions


Computers and Technology, 15.12.2020 05:20


Health, 15.12.2020 05:20




Mathematics, 15.12.2020 05:20

Mathematics, 15.12.2020 05:20

Mathematics, 15.12.2020 05:20

English, 15.12.2020 05:20









Mathematics, 15.12.2020 05:20