
Chemistry, 31.08.2019 22:30 johndiaz26
Nitric oxide, no, is made from the oxidation of nh3, and the reaction is represented by the equation 4nh3 + 5o2 ? 4no + 6h2o an 9.5-g sample of nh3 gives 12.0 g of no. the percent yield of no is :

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
Nitric oxide, no, is made from the oxidation of nh3, and the reaction is represented by the equation...
Questions




Engineering, 04.07.2019 18:10













Engineering, 04.07.2019 18:10



Engineering, 04.07.2019 18:10

 .....(1)
.....(1)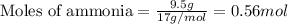
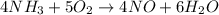
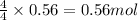 of NO
of NO




